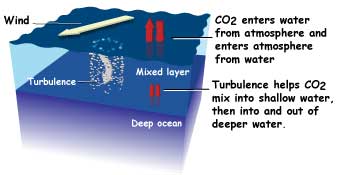
The ocean absorbs carbon dioxide from the atmosphere wherever air meets water. Wind causes waves and turbulence, giving more opportunity for the water to absorb the carbon dioxide.


The ocean absorbs carbon dioxide from the atmosphere wherever air meets water. Wind causes waves and turbulence, giving more opportunity for the water to absorb the carbon dioxide.
Fish and other animals in the ocean breathe oxygen and give off carbon dioxide (CO2), just like land animals. Ocean plants take in the carbon dioxide and give off oxygen, just like land plants. The ocean is great at sucking up CO2 from the air. It absorbs about one-quarter of the CO2 that we humans create when we burn fossil fuels (oil, coal, and natural gas.) If not for the ocean, we'd be in even worse trouble with too much CO2.
However, the ocean and everything in it are paying a price. The ocean is becoming more acidic.
What does this mean? Liquids are either acid or alkaline. Each liquid falls somewhere along a scale with acid at one end and alkaline at the other.

Normally, ocean water is less acidic than fresh water. Unfortunately, as the ocean absorbs more and more carbon dioxide from the atmosphere, it becomes more acidic. Lemon juice is an example of an acidic liquid. Toothpaste is alkaline. The ocean is slightly alkaline.
However, when the ocean absorbs a lot of CO2, the water becomes more acidic. The alkalinity of the ocean is very important in maintaining a delicate balance needed for animals--like the mussels in this picture—to make protective shells. If the water is too acidic, the animals may not be able to make strong shells. Corals could also be affected, since their skeletons are made of the same shell-like material.