Burning 6.3 pounds of gasoline produces 20 pounds of carbon dioxide.
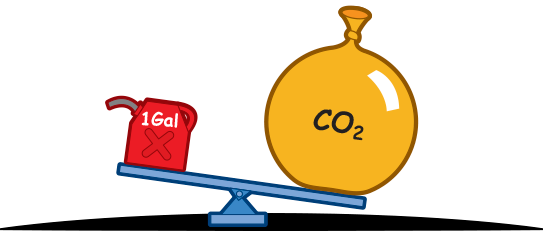
Huh!?
Here's how. Most of the weight of carbon dioxide (CO2) comes from the two oxygen atoms (the O2). Gasoline molecules are made of carbon and hydrogen atoms all bound together. When gasoline burns, the carbon and the hydrogen in the gas molecules separate. Two hydrogen atoms combine with one oxygen atom to form H2O, or water.
Each carbon atom in the gasoline combines with two oxygen atoms already in the air. This forms CO2.
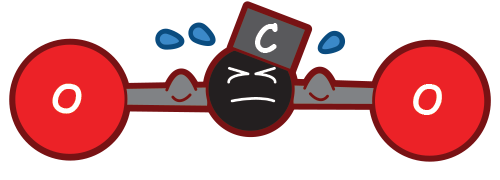
If you are curious about why the CO2 is so much heavier than the gasoline—and you like math—read on.
A carbon atom has an atomic weight of 12. This means its nucleus (center) contains 6 protons and 6 neutrons, adding up to 12. One oxygen atom has an atomic weight of 16. So each molecule of CO2 has an atomic weight of 44:
1 carbon
+ 1 oxygen
+ 1 oxygen
= carbon dioxide
12 + 16 + 16 = 44
So the total atomic weight of a molecule of CO2 is 44, which is 3.7 times more than the carbon atom alone weighs (44 divided by 12).
Next, we need to know how much of the weight of the gasoline is just the carbon. Gasoline is about 87% carbon and 13% hydrogen by weight. So the carbon in a gallon of gasoline (weighing 6.3 pounds) weighs 5.5 pounds (.87 x 6.3 pounds = 5.5 pounds). So, multiply the weight of the carbon times 3.7, which equals 20 pounds of carbon dioxide!
Just something to think about when you fill the tank of the family car!