What Is Ocean Acidification?

Ocean waves off the coast of New Zealand. Credit: Public domain
Have you ever heard someone call water "H2O"? That nickname means that water is a combination of two chemicals called hydrogen (H) and oxygen (O). All water contains hydrogen and oxygen. However, most water has a lot of other stuff in it, too.
For example, ocean water has salt in it, while water from a river or lake does not. The amount of salt is one feature, or property, of water. This property is very important to living things. Most freshwater plants and animals can’t survive if water is too salty — and most ocean life can’t survive in the fresh water of rivers and lakes.

Clownfish need to live in the salty ocean water to survive. Credit: Public domain
Water also has other properties that need to stay in balance for living things to thrive. One of those properties is called acidity.
What is acidity?
An acid is a substance that can cause certain changes when it is combined with water or a metal. Acidity is the amount of acid in water.
Lemon juice and vinegar are examples of foods that have acids in them. The acid in lemon juice is what makes it taste sour.
Acids can cause chemical reactions when they touch metal, rocks or other surfaces. The chemical reactions are known for eating away at, dissolving or damaging other substances.

Metal that comes into contact with acid can form rust on the surface. Credit: Public domain
The acidity of a substance is measured using the pH scale. A substance with a pH of 7 is considered neutral. An acid has a pH less than 7. Anything with a pH higher than 7 is called a base.
Here is a list of how everyday things measure up on the pH scale:
- 0: Battery Acid
- 1: Stomach Acid
- 2: Vinegar
- 3: Orange Juice
- 4: Tomato Juice
- 5: Coffee
- 6: Milk
- 7: Pure Water
- 8: Ocean Water
- 9: Toothpaste
- 10: Mild detergent
- 11: Window cleaner
- 12: Hair straighteners
- 13: Bleach
- 14: Drain Cleaner
What causes ocean acidification?
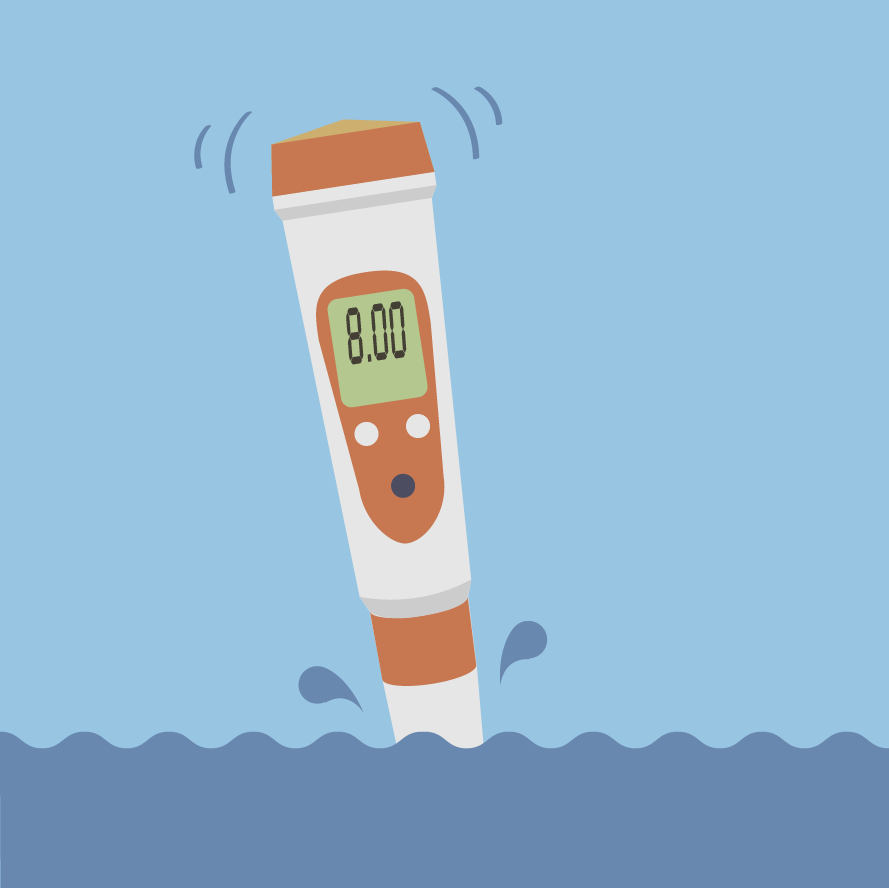
Ocean water is approximately pH 8. Credit: NASA/JPL-Caltech
As you probably noticed in the list above, ocean water has a pH of approximately 8. That means ocean water is fairly neutral — and slightly basic. However, in the past 100-200 years, scientists have observed that ocean water has become 30 percent more acidic.
Why is this happening? Well, many human activities — such as exhaust from cars and factories—release a gas called carbon dioxide into the air. Extra carbon dioxide can cause problems in the atmosphere, and it can also have an impact on the acidity of the ocean.
Extra carbon dioxide gas in the atmosphere is absorbed by the top layer of the ocean. And when carbon dioxide absorbs into water, it makes the water more acidic.
Why does ocean acidification matter?

Coral reefs are important to ocean ecosystems. Credit: Greg McFall/NOAA
Just like acids can cause rust and other damage to surfaces on land, acidic ocean water can affect surfaces underwater. Acids can break down the shells of animals that live in the sea. Because ocean water has become more acidic, some animals — like certain oysters and clams — are having difficulty in making or keeping their shells.
For example, acidic ocean water can cause coral to grow more slowly and weaken coral reefs. These reefs are an important home for many living things. Their health is essential to many ecosystems.
How is NASA studying ocean acidification?
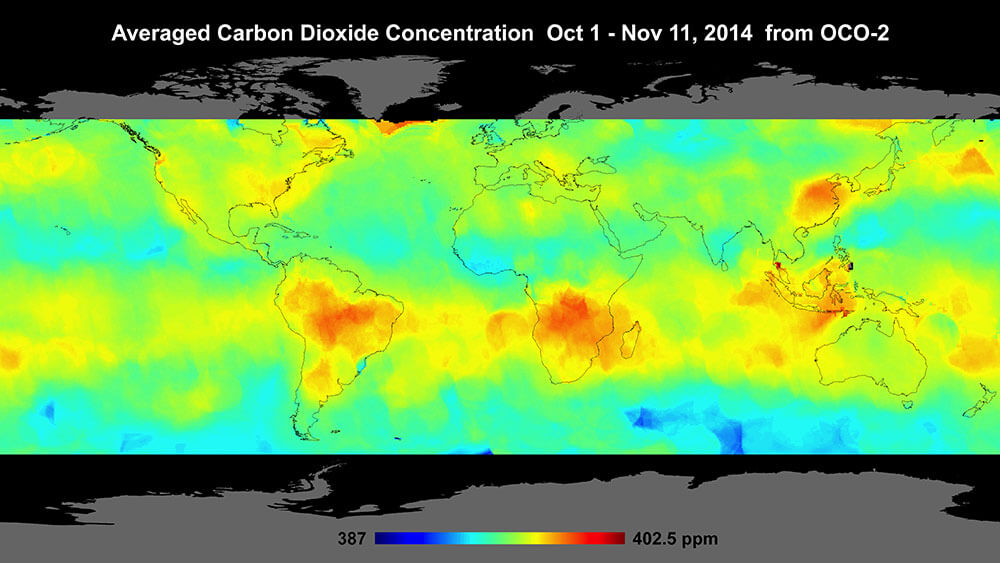
The red areas in this map are spots with high levels of carbon dioxide in the atmosphere. The map was created using information collected by NASA's OCO-2 satellite. Credit: NASA/JPL-Caltech
NASA satellites are orbiting and collecting information about Earth all the time. One satellite, called Orbiting Carbon Observatory-2 (OCO-2), collects information about carbon dioxide in our atmosphere while it circles Earth.
Scientists can use these measurements to better understand the relationship between carbon dioxide and changes in the ocean’s acidity.
Related NASA Missions